Organska kemija: sinteza, struktura in aplikacija
P1-0230
Trajanje: 1.1.2022 - 31.12.2027
Vodja:
prof. dr.
Košmrlj Janez
Kemijska sinteza in posledično dostopnost do najrazličnejših sintetičnih spojin je koristila družbi na številne načine v vseh obdobjih našega razvoja. Vendar pa izziv kemije danes ni več zgolj sinteza ciljnih molekul, ampak predvsem kakšne so možnosti njihove uporabe in kako te spojine pripraviti na praktičen, učinkovit in okolju prijazen način. Raziskovalno delo našega programa, ki vključuje področja organske, anorganske, koordinacijske, farmacevtske in medicinske kemije, sledi opisanim izzivom. Ukvarjamo se z odkrivanjem in uporabo novih, učinkovitejših in trajnejših reakcij ter sinteznih metodologij tako v smeri razvoja in priprave katalizatorjev, drugih funkcionalnih molekul in biološko pomembnih spojin. Nepogrešljivo izhodišče je poznavanje mehanizmov kemijskih reakcij, temelja kemijske znanosti, ki omogoča racionalno zasnovo reakcijskih pogojev, zmanjšanje stroškov izhodnih spojin ter zmanjšanje tvorbe problematičnih odpadkov.
Naš program obsega tri glavne raziskovalne tematike: študij mehanizmov kemijskih reakcij, sintezo in katalizo ter praktično uporabo pridobljenih znanj.
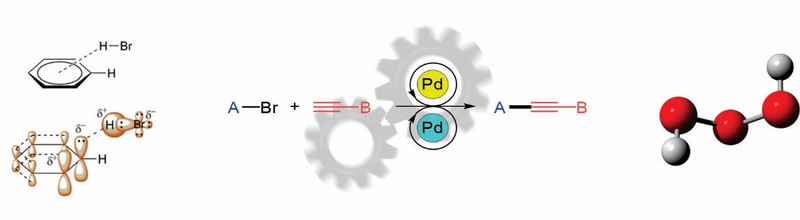
Razumevanje kemijskih reakcij na mehanističnem nivoju je ključnega pomena za obvladovanje in uporabo kemijskih procesov, saj je le tako možno racionalno načrtovanje in optimizacija sintez, kakor tudi odkrivanje novih reakcij. Ukvarjamo se z reševanjem ključnih mehanističnih vprašanj mnogih reakcij, vključno s tistimi, ki potekajo pod klasičnimi pogoji, kot tudi s takimi, ki potekajo pod pogoji (organo)katalize ter z redoks reakcijami in reakcijami spajanja [PCCP 2021]. Pred kratkim smo na novo definirali mehanizem Sonogashirove reakcije, ki poteka brez prisotnosti bakrovih zvrsti in je dotlej temeljil na napačni predpostavki uveljavljenega mehanizma izpred skoraj petih desetletij [NatChem 2018]. Na področju reakcij spajanja je naš cilj razširiti kemijo še na druge kovine, kar lahko privede do odkritja novih reakcij, podobno kot smo nedavno odkrili nov katalitski sistem paladij-paladij [ChemComm 2016, OL 2020]. Odgovorili smo tudi na izzive katalitske Mitsunobu reakcije [OL 2016, Chem Sci 2016] in sinteze zelo reaktivnih in nestabilnih zvrsti, kot so vodikov trioksid (HOOOH) in njegovi derivati [ACIE 2015]. Ti so pogosti intermediati pri oksidacijah in so vse bolj prepoznani v številnih kemijskih, biokemijskih, atmosferskih in okoljskih procesih [ChemRev 2013]. Posvečamo se tudi številnim drugim strukturnim, sinteznim in mehanističnim vprašanjem ter njimi povezanimi procesi in tehnikami [J Chem Inf Model 2021, PCCP 2021].
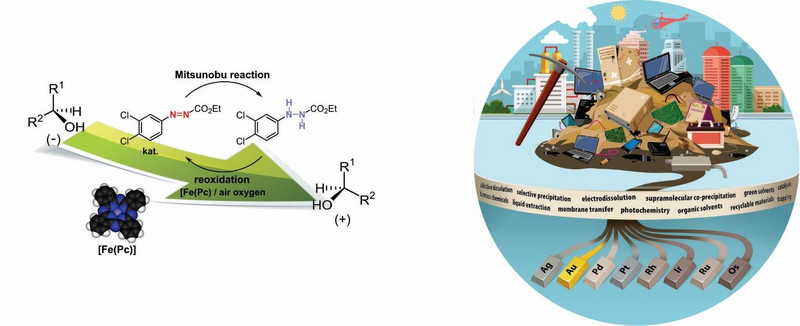
Pomemben del naših raziskav namenjamo zeleni kemiji, ki predstavlja eno bistvenih usmeritev k zdravju in okolju bolj prijaznim sintezam, s končnim ciljem doseči sonaravni in trajnostni razvoj. Številni izzivi na tem področju, s katerimi se ukvarjamo, so na primer zamenjava lahko hlapnih, vnetljivih ali strupenih organskih topil ter škodljivih reagentov z bolj prijaznimi alternativami. Primer je pretvorba tiolov v sulfonil halogenide z uporabo zračnega kisika kot končnega oksidanta, oksidacija sulfidov do sulfonov z vodikovim peroksidom brez uporabe topil in katalizatorjev ter redukcija sulfoksidov v sulfide v odsotnosti organskega topila [Green Chem 2017, GCLR 2020]. Drugo pomembno področje v okviru zelene kemije je recikliranje ter ponovna uporaba katalizatorjev in žlahtnih kovin [ACIE 2022, ACIE 2022]. Razvili smo katalitsko Mitsunobu reakcijo z azo reagenti, ki jih je mogoče reciklirati [Chem Sci 2016].
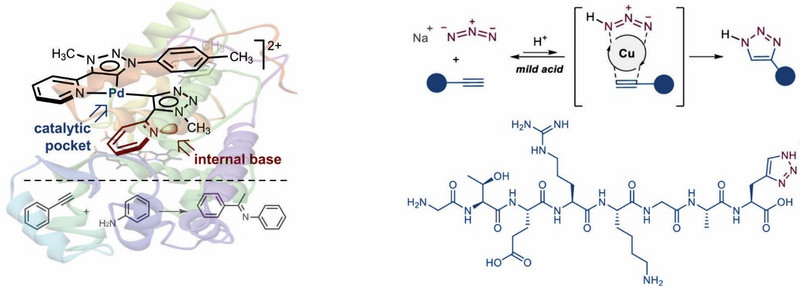
Na učinkovitost reakcije je mogoče vplivati s katalizo. Katalizirane reakcije so danes ključne pri proizvodnji več kot 80% spojin, s katerimi se srečujemo v vsakdanjem življenju. Katalizatorji in katalitski procesi so povezani s približno 30% celotnega BDP evropskega gospodarstva. Nedavno smo razvili N-heterociklični karbenski (NHC) ligand na osnovi triazola z mezoionsko (MIC) strukturo – PyMIC – z bistveno izboljšanimi lastnostmi glede na druge ligande [ChemComm 2016]. PyMIC spadajo med nove SMART (»Switchable, Multifunctional, Adaptable, oR Tuneable«) ligande, ki omogočajo razvoj izjemno aktivnih katalizatorjev na osnovi kovin prehoda [OL 2020, OL 2020]. Sem spadajo Ru-, Os- in Ir-katalizatorji za selektivne oksidacije in redukcije ter Pd-kompleksi za reakcije spajanja (npr. reakciji Suzuki-Miyaura in Sonogashira), ki delujejo v vodi kot edinem topilu in v prisotnosti zraka. Kompleks Pd-PyMIC kaže encimom podobno reaktivnost pri katalitskem hidroaminiranju alkinov. Edinstvene lastnosti kompleksov s PyMIC, pa tudi z drugimi ligandi, kot so npr. azokarboksamidi, imajo zato potencial tudi v mnogih drugih katalitskih reakcijah in so v središču našega raziskovalnega dela [Organometallics 2021].
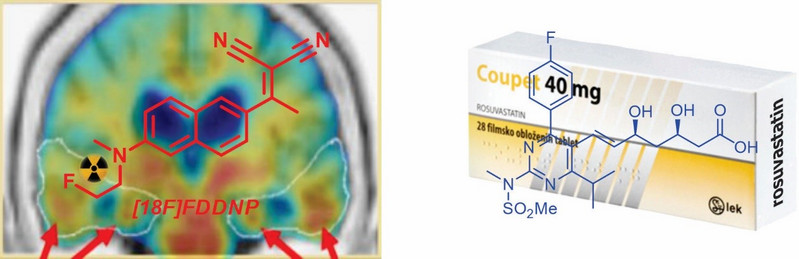
Ena izmed naših aplikativnih raziskovalnih tem je povezana z razvojem molekularnih sond za zgodnje odkrivanje Alzheimerjeve bolezni. Alzheimerjeva bolezen postaja eden izmed vodilnih problemov sodobne medicine, saj ima izjemno negativen vpliv na kvaliteto življenja bolnikov in njihovih družin, znatno pa obremenjuje tudi nacionalne proračune za zdravstveno varstvo po vsem svetu. Naš zgodnji prispevek k diagnostiki Alzheimerjeve bolezni je povezan z razvojem radioaktivne sonde [18F]FDDNP za in vivo slikanje patoloških proteinskih agregatov s pozitronsko emisijsko tomografijo (PET) [PNAS 2012]. Kot alternativa in vivo slikanju, ki zahteva izjemno drago specializirano infrastrukturo in predstavlja veliko tveganje za zdravje bolnikov, so naša trenutna prizadevanja osredotočena na pametne molekularne sonde za zgodnje napovedovanje Alzheimerjeve bolezni na podlagi ex vivo detekcije ustreznih biomarkerjev v telesnih tekočinah [JMC 2017]. Poleg tega se ukvarjamo z razvojem novih biološko pomembnih molekul s citotoksičnimi [Chem Eur J 2014, Organometallics 2019] in antibakterijskimi lastnostmi [EJMC 2017, Chem Biol Drug Des 2017]. V sodelovanju z razvojnima centroma Sandoz Austria in Sandoz Slovenija smo razvili popolnoma stereokontrolirano aldolno reakcijo na kiralnih β-aminokislinah [OL 2015] in laktonsko pot do statinov z uporabo Wittigove reakcije [JOC 2010].
Razvoj novih tehnik, metod, katalizatorjev, intermediatov in uporabnih spojin je ključnega pomena za trajnostni napredek raziskovalno usmerjene kemijske industrije in s tem sodobne družbe. V tem okviru se v naši programski skupini trudimo za razvoj novih znanj, vzpostavitev čim širše mreže sodelovanj z domačimi in mednarodnimi inštitucijami, prenos znanja iz akademskega okolja v industrijo (in obratno), s čimer nenazadnje prispevamo tudi k razvoju visokokakovostnih mladih znanstvenikov. Več o delu naše programske skupine si lahko preberete na Košmrlj Group, najnovejšemu dogajanju pa sledite tudi na Twitter profilu skupine @KosmrljGroup. Prijazno vabimo vse zainteresirane, da nas kontaktirajo in se nam pridružijo pri našem delu.

